Accelerating the path from discovery to drug
An industry partner is helping Temple scientists bring treatments to market faster.

All drugs on the market today originated with someone’s curiosity. Wondering how electricity could affect bacteria led to the discovery of Cisplatin, the first drug for testicular cancer; while a mystery surrounding dying cows led to the development of the anticoagulant Coumadin. But to get from curiosity to drug on the shelf, it can take up to 10 years and $2.6 billion. And that’s only if someone recognizes the potential, funders take notice and commercialization looks feasible.
On top of this, the drug development environment has changed dramatically over the past decade: Federal research funding is stagnant due to a variety of economic and political pressures, and the discovery of blockbuster drugs, which fueled pharmaceutical progress for so long, has languished largely because the model was unsustainable.
As research universities and the pharmaceutical industry adapt to an altered landscape, they’re looking for new ways to bring promising treatments to people faster.
From an infected root canal
For several decades now, Roy Stevens has investigated stubborn root canal infections that refuse to heal despite treatment. Driving him is curiosity about the bacteria responsible for such stubborn infections, Enterococcus faecalis, and how it operates. Along the way, he discovered that one of the strains of the bacteria is infected by a virus.
“So here we had a virus that came out of an infected root canal and nobody knew it,” said Stevens, professor of endodontics at Temple’s Kornberg School of Dentistry.
He began to study the virus not only to better understand the biology of an infected root canal, but also because he knew there was another aspect to viruses that could prove useful.

Stopping the march of a progressive disease
Next door on Broad Street at Temple’s Health Sciences Center another scientist was diving deeply into the biology of a different disease.
Chronic Obstructive Pulmonary Disease (COPD), largely caused by smoking, affects more than 24 million people in the U.S. and is predicted to become the third-leading cause of death worldwide by 2030, according to the World Health Organization. As time goes on, breathing compromised by COPD becomes more and more difficult. Right now, there’s no way to cure the disease or slow it down, only treatments to alleviate symptoms like tightness in the chest.
Salim Merali has long been intrigued by the lung. A complex organ, the lung is made up of eight different types of cells, compared to two or three in most other organs.
“While this makes it harder to study, I enjoy such challenges,” said Merali, professor of pharmaceutical science in Temple’s School of Pharmacy. “I’m also interested in COPD because I’m from Africa, where smoking is quite prevalent.”
Merali was curious about the progression of COPD and what that might reveal about possible treatments. Early in his career, he became proficient in proteomics, which allows the study of proteins—important building blocks found in all cells.
He identified the proteins in COPD cells and compared them to proteins in cells from people without COPD. His analysis revealed that the proteins from COPD patients had changed, and one protein in particular had changed significantly.
“From that point, we focused on the protein that had changed the most. Intriguingly, that protein was not only inside the cell, but also outside, coursing through the bloodstream,” said Merali.
He discovered that when the changed protein was added to a normal lung cell, it killed the cell.
This means that people with COPD are producing a protein that destroys lung cells, which is how the disease progresses. Over time, sufferers produce more and more of these proteins and their symptoms worsen.
If the protein could be stopped from attacking other cells, the progression of the disease would stop, Merali realized. Maybe someday this technology could even be used to prevent COPD altogether.
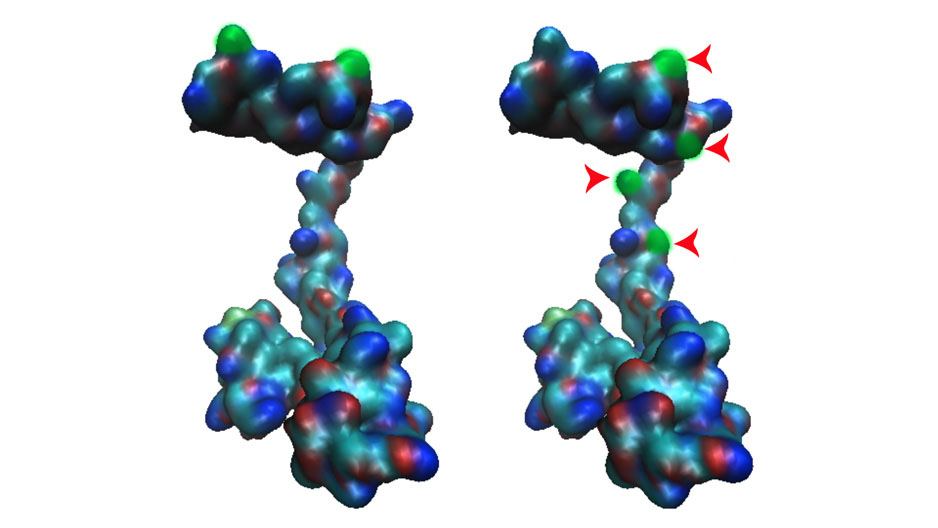
Photography by: Courtesy of Salim Merali
Salim Merali discovered a protein (left) that was altered in patients with COPD (right).
Zeroing in
Like Merali, Stevens was zeroing in on a potential target.
He’d discovered that the bacteria responsible for the treatment-resistant root canal infections contained a virus. Could this virus be responsible for the resistance? And if it was, could it somehow be used to fight other drug-resistant infections?
Infections that have adapted to antibiotics and now ignore them constitute an emerging health threat worldwide. Experts point to overprescribing and improper patient use as the main drivers of this growing antibiotic resistance. The Centers for Disease Control estimate that each year at least 2 million people are affected by and 23,000 people die from antibiotic-resistant infections.
Stevens’ idea of using a virus to treat disease, called phage therapy, has been around for almost 100 years. In the early 1900s, the scientific community hotly pursued it as a treatment for infections. But when antibiotics hit the scene, phage therapy lost its appeal, except in the former Russia and Eastern Europe—countries that didn’t have access to Western medicines. Over the past several decades, phage therapy has been developed for burns and wound treatment, as well as food-borne infections.
Stevens knew there was another compelling reason to revisit phage therapy as a potential treatment for antibiotic-resistant infections.
“Phage therapy is very selective,” he said. “The viruses have no effect on human cells beyond the bacteria at which they’re aimed. When you develop a virus to treat a specific kind of infection, it won’t damage our microbiome [the community of microorganisms that make up every living being].”
The virus by itself wouldn’t be useful, though. Stevens and his team had to figure out how to develop it into an infection fighter.
They took an approach no one else had tried yet. Based on a series of genetic studies, they decided to re-engineer the virus. With each success, they were able to go further, largely through support from a National Institutes of Health (NIH) grant. Eventually, though, they ran out of money and the prospect of more federal funding looked dim.
Salim Merali was facing a similar dilemma.

Photography by: Joseph V. Labolito
Salim Merali (left) and his research team want to see if they can prevent the progression of COPD by targeting a protein.
His team had already identified a protein involved in the progression of COPD and considered it a promising target for treatment. But there was still work to do, and they needed financial backing.
As promising as their work was, both Stevens and Merali were still in the early stages of their research. It wasn’t yet clear if their targets could attract interest as marketable products. Plus, it was becoming more and more difficult to attract the interest of the NIH, which is more focused on hypothesis-based research and the biology of a disease than it is on drug development.
It was time to look beyond the usual funding sources.
Pharma Germinator to the rescue
Four years ago, Steve Nappi’s first proposal to Janssen Research & Development’s Ken Kilgore didn’t quite fly. Nappi, associate vice president of technology commercialization and business development at Temple, was trying to sell a treatment for infections that came out of Temple’s Dental School.
“We don’t do dental,” Kilgore, Scientific Director, Immunology Discovery, replied, referring to Janssen’s five areas of strategic focus.
But Janssen ultimately decided to take a closer look because infectious disease was one of their strategic areas of focus.
The project started with Roy Stevens meeting Janssen scientists and strategists, face-to-face, at the pharmaceutical company’s research and development (R&D) hub in Spring House, Pennsylvania. This meeting turned out to be critical.
The Janssen scientists and strategists had the chance to probe, ask questions and get a sense of what a partnership with Stevens would be like.
Kilgore said he gets goosebumps when he recalls that initial meeting with Stevens.

Everyone was impressed with the Temple professor and envisioned an easy rapport and fruitful relationship. They also saw enormous potential for an innovative product.
“We saw that it went beyond infections and was really a platform technology [a foundation for multiple applications],” said Christine McCauley, a scientist in Immunology Discovery at Janssen R&D. “[Stevens’] expertise was in the technology that altered the phage to increase the specificity making it much more useful as a pharmaceutical agent,” McCauley continued.
“The shaping of an application is so tremendously important,” said Nappi. “We come in with a dashboard of possibilities, not knowing what the best route is to commercialization. Janssen helps us focus on what will have greatest impact in the marketplace.”
Temple and Janssen collaborating in this way was spurred by the Pharma Germinator, an initiative that started with state funding to BioStrategy Partners to create a program for advancing drug development. The Janssen Immunology group named the program as the Germinator and it has since evolved to include six research institutions including Thomas Jefferson University and Penn State University.
“The Germinator is a way to access existing technology coming out of universities that others haven’t explored yet,” said Christine McCauley.
It’s much faster than what pharmaceutical companies used to do, which was pore over scientific literature for leads. Another problem with that strategy? Everyone else was doing the same thing.
The elegance of the Germinator lies in the union of two groups with different missions: Research universities are about discovery, and Janssen is focused on advancing innovative science to deliver novel products. But they do have some common ground—both long to find ways to cure disease and improve lives. The Germinator helps them to meet in the middle.
“Universities excel at early stage research when market rewards are the most distant aspect of developing a strategy,” said Michele Masucci, vice president of research at Temple. “This allows scientists to be more creative, but we don’t have the amplified view industry has to be able to pick a project from a market perspective. Stevens needed someone to recognize the potential of his research and now he is involved in multiple industry collaborations, which is really rewarding to see evolve.”

Photography by: Ryan Brandenberg
Stevens’ (left) idea of using a virus to treat disease, called phage therapy, has been around for almost 100 years.
On the road to market
Janssen has since supported several of Stevens’ projects, which focus on both the original bacteria and an additional strain. The team believes they have a potential game-changer for the treatment of antibiotic-resistant infections.
The work caught the attention of the technology community, and last year Temple launched Lytphage—recognized as one of the top university startups in the country by the National Council of Entrepreneurial Tech Transfer. The company will concentrate on developing one of those viruses as a product.
Kilgore estimated that reaching the development stage would take about two years. The team has started conversations with the FDA and is examining how to get this treatment manufactured.
Merali explained how the Pharma Germinator goes beyond bringing in financing to continue a research project. Temple scientists also get the expertise of Janssen colleagues.
“Their experts are very knowledgeable in moving drugs from the preclinical to clinical stage,” said Merali. “We as basic scientists tend to focus too much on mechanisms. They want to move forward in the direction of drug development. They not only understand the overall biology of the target for the drug, but also how the drug might affect the target.”
The COPD technology that Merali developed is now in clinical studies and he and Nappi are looking for commercial partners with which to launch a startup.
Win-win
So far, Temple has advanced seven projects and three startups through the Germinator. In addition to COPD and infection, scientists are investigating promising treatments for rheumatoid arthritis, inflammatory bowel disease and psoriasis.
Though accelerating the path to commercialization is the shining light on the horizon, both Temple and Janssen are delighted with some unexpected rewards along the way.
The Germinator has provided a foundation on which to build exceptional relationships. So much so, Kilgore reports, that Temple has become an outstanding collaborator for Janssen’s, especially with the close Philadelphia life science ties.

“This is not just us telling scientists what to do,” said Kilgore. “This is real interaction, where everyone is learning. It’s become a development tool for our staff, because they learn how to work with external partners. We as an industry haven’t always been very good at that.”
The Janssen team is all in. In fact, Kilgore said that there aren’t enough spots for all the Janssen staff members who want to participate.
At Temple, Masucci credits the collaboration with providing a blueprint for faculty and industry partnerships going forward.
“This is a great example of how you can build a rapport and an understanding to move forward in tandem,” Masucci said. “Learning to not only advance a technology, but also looking at the horizon to see what’s coming up from industry’s perspective is also of value for our faculty. We couldn’t have done it on our own.”